http://www.chem4kids.com/files/react_acidbase.html
http://www.chemtutor.com/acid.htm
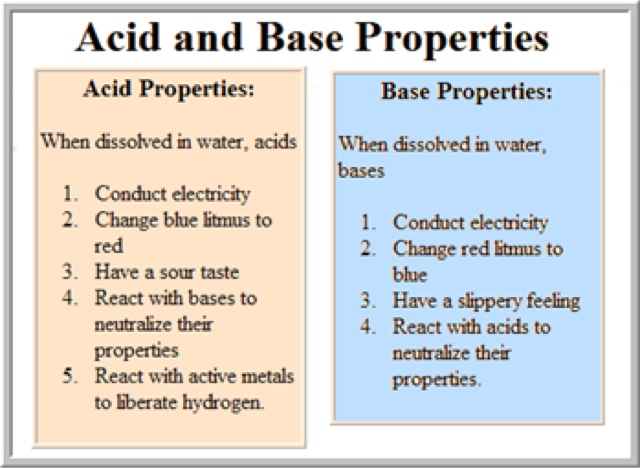
BASES: taste bitter and feel slippery
ARRHENIUS ACIDS AND BASES
-Arrhenius acids are those species that produce hydrogen ions in solution (H+)
-Arrhenius bases are those species that produce hydroxide ions in solution (OH-)
HCl-->H+ + Cl- (strong acid)
NaOH-->Na+ + OH- (strong base)
WATER CAN BE AN ACID OR A BASE (amphoteric)
BRØNSTED-LOWREY ACIDS AND BASES
Brønstead-Lowery acids donate a proton (H+)
Brønstead-Lowery bases accept a proton (H+)
HCl + H2O --> H3O+ + Cl-
Acid-Conjugate Base Pair [HCl and Cl-]
Base-Conjugate Acid Pair [H2O and H3O]
EX: HClO4 and ClO4- because H+ + ClO4-