http://www.chem4kids.com/files/react_acidbase.html
http://www.chemtutor.com/acid.htm
BASES: taste bitter and feel slippery
ARRHENIUS ACIDS AND BASES
-Arrhenius acids are those species that produce hydrogen ions in solution (H+)
-Arrhenius bases are those species that produce hydroxide ions in solution (OH-)
HCl-->H+ + Cl- (strong acid)
NaOH-->Na+ + OH- (strong base)
WATER CAN BE AN ACID OR A BASE (amphoteric)
BRØNSTED-LOWREY ACIDS AND BASES
Brønstead-Lowery acids donate a proton (H+)
Brønstead-Lowery bases accept a proton (H+)
HCl + H2O --> H3O+ + Cl-
Acid-Conjugate Base Pair [HCl and Cl-]
Base-Conjugate Acid Pair [H2O and H3O]
EX: HClO4 and ClO4- because H+ + ClO4-


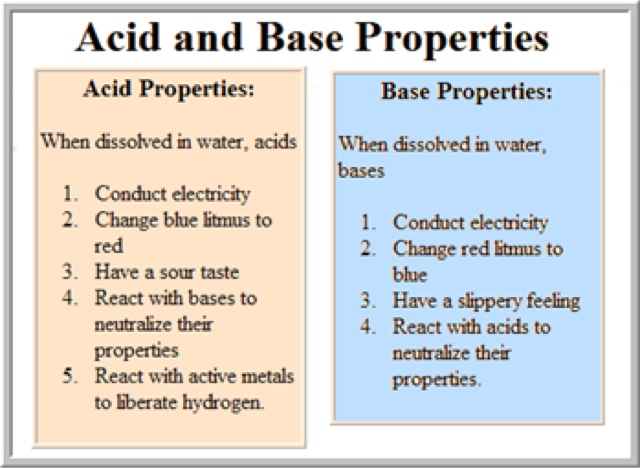
Man, if only B-L was as easy to remember as Arrhenius. Thanks for the diagram by the way, I have a feeling that there will be something like this on the quiz tomorrow.
ReplyDeleteGreat review over acids and bases! The pictures are also very helpful!
ReplyDeleteTotal activity of conjugate against immobilized avidin >95%. Contains approx. two moles biotin to one mole of lysozyme. Lysozyme Biotin-Caproyl
ReplyDeleteVery informative article, Which you have shared here about the Acid and Bases. After reading your article I got very much information about the Acid and Bases and it resolved many of my doubts. Thanks for sharing this article here. example of acid and bases
ReplyDelete