I don't think that I did so well on the Gas Laws quiz. I know that for the test, I'll need to study the different laws closer, and notice the differences between them. I missed a day, and totally had no clue what STP was, which seems pretty important now.
STP
Tuesday, May 10, 2016
Air Bag Lab
We had to construct an air bag... one that would save our passenger's life but also not hurt the passenger from being too full of air.
Here's what we decided to do...
http://chemistry.about.com/od/chemicalreactions/f/What-Is-The-Equation-For-The-Reaction-Between-Baking-Soda-And-Vinegar.htm
Here's what we decided to do...
- Find maximum volume of the bag
- Fill bag with water
- pour H2O into a graduated cylinder--- Volume H2O=Volume CO2
- Calculate to determine amount of NaHCO3 needed
- Volume from step 1, convert to L
- Looking for NaHCO3... n=PV/RT
- solve for moles of CO2
- use stoich to find grams of NaHCO3
- Baking soda=vinegar 1:1 molar ratio
- Use D of vinegar to find volume of vinegar
- x.20 because vinegar is only 5% acetic acid
- Multiply measurements by .65 to not completely fill up the bag (or explode it)
http://chemistry.about.com/od/chemicalreactions/f/What-Is-The-Equation-For-The-Reaction-Between-Baking-Soda-And-Vinegar.htm
PVNRT
PV=nRT
R=universal gas constant .0821 L atm/ mol K
EX. Calculate the pressure in atm exerted by 1.82 mol of Sulfur Hexaflourine gas in a steel vessel with a volume of 5.43 L at 69.5 degrees C (+273.15)
PV=nRT
P= nRT/V
(1.82 x .0821 x 342.65)/ 5.43
=9.43 atm
http://www.phscale.net/idealgaslaw.htm
http://www.westfield.ma.edu/cmasi/gen_chem1/Gases/ideal%20gas%20law/pvnrt.htm
R=universal gas constant .0821 L atm/ mol K
PV=nRT
P= nRT/V
(1.82 x .0821 x 342.65)/ 5.43
=9.43 atm
http://www.phscale.net/idealgaslaw.htm
http://www.westfield.ma.edu/cmasi/gen_chem1/Gases/ideal%20gas%20law/pvnrt.htm
Measuring Pressure
Common units of pressure:
688mmHg x (1atm/760mmHg) = .905 atm
http://www.sengpielaudio.com/ConvPress.htm
- Pascal Pa
- Avg Air pressure at sea level 101,325
- Kilopascal kPa
- Avg Air pressure at sea level 101.325
- Atmosphere atm
- 1 exactly
- Millimeters of Mercury mmHg
- 760 exactly
- Inches of Mercury inHg
- 29.92
- Torr torr
- 760 exactly
- Pounds per square inch psi
- 14.7
688mmHg x (1atm/760mmHg) = .905 atm
http://www.sengpielaudio.com/ConvPress.htm
Boyle's Law
TELLS THE RELATIONSHIP BETWEEN PRESSURE AND VOLUME IN AN INVERSE RELATIONSHIP
P1V1=P2V2
Ideal gas at low pressure holds strictly to this relationship
http://www.wisegeek.org/what-is-boyles-law.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/gases-5/gas-laws-51/boyle-s-law-volume-and-pressure-254-8360/
P1V1=P2V2
Ideal gas at low pressure holds strictly to this relationship
http://www.wisegeek.org/what-is-boyles-law.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/gases-5/gas-laws-51/boyle-s-law-volume-and-pressure-254-8360/
Calculating Heat...
Q=MCΔT
Q= Heat in Joules
M= Mass in grams
C= Specific Heat
ΔT= Change in Temperature
EXAMPLE:
Calculate the amount of energy in joules required to heat 454 grams of water from 5.4 degrees C to 98.6 degrees C. Calculate the amount of energy in calories, too.
Q=?
M=454 g
Tinitial=5.4 degrees C
Tfinal=98.6 degrees C
c= 4.184 J/gdegC
Q=454x4.184x(98.6-5.4)
Q=1.77x10^5 Joules or 177 KJ
https://www.youtube.com/watch?v=4RkDJDDnIss
http://www.wikihow.com/Calculate-Specific-Heat
http://chemistry.about.com/od/workedchemistryproblems/a/Specific-Heat-Example-Problem.htm
Q= Heat in Joules
M= Mass in grams
C= Specific Heat
ΔT= Change in Temperature
EXAMPLE:
Calculate the amount of energy in joules required to heat 454 grams of water from 5.4 degrees C to 98.6 degrees C. Calculate the amount of energy in calories, too.
Q=?
M=454 g
Tinitial=5.4 degrees C
Tfinal=98.6 degrees C
c= 4.184 J/gdegC
Q=454x4.184x(98.6-5.4)
Q=1.77x10^5 Joules or 177 KJ
https://www.youtube.com/watch?v=4RkDJDDnIss
http://www.wikihow.com/Calculate-Specific-Heat
http://chemistry.about.com/od/workedchemistryproblems/a/Specific-Heat-Example-Problem.htm
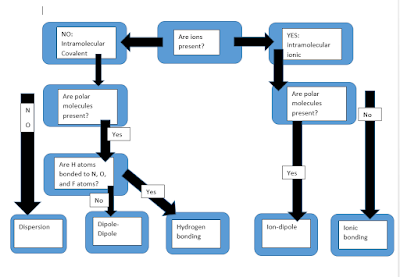
Intra/Inter Molecular Forces
I made this diagram to help with intramolecular and intermolecular forces... hope it's helpful...
http://chemed.chem.purdue.edu/genchem/topicreview/bp/intermol/intermol.html
Biodiesel Research...
Compilation of Biodiesel Research...
http://www.biodiesel.com/biodiesel/what-is-biodiesel/
http://www.fueleconomy.gov/feg/biodiesel.shtml
Click here for my favorite study music
- renewable
- clean-energy
- Blends of B20 can be used in a diesel engine without need for modifications
- IS registered with the Environmental Protection Agency in the United States
- Can be blended with petroleum or used purely
- Statistically safer than petroleum diesel
- made with natural vegetable oils and fats
http://www.biodiesel.com/biodiesel/what-is-biodiesel/
http://www.fueleconomy.gov/feg/biodiesel.shtml
Click here for my favorite study music
Monday, May 9, 2016
Making Biodiesel
WE MADE OUR OWN BIODIESEL
In class, we used Chickfila frier oil to create renewable biodiesel!
How to make biodiesel with used cooking oil
Everyone should make their own biodiesel.
http://biodiesel.org/
In class, we used Chickfila frier oil to create renewable biodiesel!
How to make biodiesel with used cooking oil
Everyone should make their own biodiesel.
http://biodiesel.org/
BIODIESEL
Biodiesel Video!!
Biodiesel is a cleaner fuel, and better for the environment. Our class made biodiesel videos to help spread the word about the renewable resource. Check out our videos!
OUR biodiesel video
another informative video...
This one just made us laugh :)
Biodiesel is a cleaner fuel, and better for the environment. Our class made biodiesel videos to help spread the word about the renewable resource. Check out our videos!
OUR biodiesel video
another informative video...
This one just made us laugh :)
Covalent Bonds
Result of sharing of electrons by two nonmetal atoms
BOTH atoms fill their octet (H exception)
Octet is satisfied when the two atoms are combined
http://quatr.us/chemistry/atoms/covalent.htm
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds
Covalent Bonding Video
BOTH atoms fill their octet (H exception)
Octet is satisfied when the two atoms are combined
http://quatr.us/chemistry/atoms/covalent.htm
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds
Covalent Bonding Video
Bonding
Electron Dot Formulas
-includes bonded and unbonded electron pairs
1) Calculate the total number of valence electrons by adding all of the valence electrons for each atom in the molecule
2) Divide the total valence electrons by 2 to find the number of electron pairs in the molecule
3) Surround the central atom with 4 electron pairs
Use the remaining electron pairs to complete the octet around the other atoms. H is the only exception, which only needs 2 electrons
4) Electron pairs that are shared by atoms are called bonding electrons
Others used to complete octets are lone pairs
5) If there are not enough electron pairs to provide each atom with an octet, move a nonbonding electron pair between two atoms that already share an electron pair
-includes bonded and unbonded electron pairs
1) Calculate the total number of valence electrons by adding all of the valence electrons for each atom in the molecule
2) Divide the total valence electrons by 2 to find the number of electron pairs in the molecule
3) Surround the central atom with 4 electron pairs
Use the remaining electron pairs to complete the octet around the other atoms. H is the only exception, which only needs 2 electrons
4) Electron pairs that are shared by atoms are called bonding electrons
Others used to complete octets are lone pairs
5) If there are not enough electron pairs to provide each atom with an octet, move a nonbonding electron pair between two atoms that already share an electron pair
Subscribe to:
Comments (Atom)

.jpg)