Acid Base Reactions
In an Acid Base Reaction, the driving force is the production of water. These reactions will produce a salt and water as products. Salt= cation of base and anion of an acid
A solution of aq nitric acid and aq potassium hydroxide are mixed in a titration
Molecular: HNO3 (aq) + KOH (aq) --> KNO3 + H2O (l)
Net: H+ (aq) + OH- (aq) --> H2O (l)
Click here for extra help!
Friday, November 27, 2015
Monday, November 23, 2015
Chemical Reactions
Chemical Reactions
Sensory Clues a change is taking place
Sensory Clues a change is taking place
- color has changed
- a solid forms
- bubbles form (in a liquid)
- Heat and/or a flame is produced, or heat is absorbed
Heat is Enthalpy = H
Delta H = Change in enthalpy
CHEMICAL REACTION LABS!
These are the pictures of products from our chemical reactions lab on Friday.
Friday, November 13, 2015
Empirical Formulas...
Empirical Formulas
What is % Composition of C10H14O?
Calculate the mass of H in 25.4 g of C10H14O.
C= 10 x 12.01 = 120.1
H= 14 x 1.01 = 14.14
O= 1 x 16 = 16.00
120.1 + 14.14 + 16.00 = 150.24 g/mol
%C= (120.1/150.24)(100)= 79.94% C
%H= (14.14/150.24)(100)= 9.412% H
%O= (16.00/150.24)(100)= 10.65% O
(9.412%)(25.4 g)= 2.39 g H
MOLECULAR FORMULAS
-whole number multiple of empirical formula
What is % Composition of C10H14O?
Calculate the mass of H in 25.4 g of C10H14O.
C= 10 x 12.01 = 120.1
H= 14 x 1.01 = 14.14
O= 1 x 16 = 16.00
120.1 + 14.14 + 16.00 = 150.24 g/mol
%C= (120.1/150.24)(100)= 79.94% C
%H= (14.14/150.24)(100)= 9.412% H
%O= (16.00/150.24)(100)= 10.65% O
(9.412%)(25.4 g)= 2.39 g H
MOLECULAR FORMULAS
-whole number multiple of empirical formula
- ratio of masses
-Can be the same as the empirical formula
EX: P4O10
EMPIRICAL FORMULAS
-lowest whole number ratio of elements in a compound
-start with % composition and work to moles
Thursday, November 12, 2015
Formula of a Chloride Lab
Formula of a Chloride Lab
Procedure:
Procedure:
- Determine the mass of a clean, dry 100 mL beaker to the nearest 0.001 g and record this mass on the data table
- Have your instructor place a small amount of zinc into the beaker. Then determine the mass of the beaker plus zinc and record in the data table.
- Using a graduated cylinder to measure, add 10 mL 3M HCl. Using a hot plate, gently heat the mixture until all of the zinc has dissolved
- Using a Bunsen burner, heat the solution until all of the water and excess HCl have been boiled away. Stop the heating as the last bit of liquid disappears. Do not continue heating after it has boiled to dryness as the compound will begin to decompose.
- Cool for several minutes and then determine the mass of the beaker and contents.
 |
| Be careful when working with Chlorine gas... You might end up not feeling well and watching the lab from the hall. |
Friday, November 6, 2015
Hydrated Compounds
Hydrated Compounds...
Have water molecules as part of their chemical formula
Contribute to the crystalline structure of the compound
HYDRATED vs ANHYDRIDES
Hydrated end up being ANHYDRIDES, which are chemicals that used to be hydrated.
Ordered (how they're arranged in space)=crystalline
What is the value of "n" for the hydrate of Magnesium Chloride H2O?
Mass of crucible = 22.130 g
Mass of crucible + hydrate = 25.290 g
Mass of crucible + contents after heating = 23.491 g
25.290 g (Cruc + Hyd) - 22.130 g (Cruc) = 3.160 (Hydrate)
23.491 g (after heating) - 22.130 g (crucible) = 1.361 g (Anhydrous)
25.290 g (hydrate) - 1.361 (anhydrous)
= 1.799 g H2O driven off
1.799 g H2O x 1 mol H2O = .09983 mol H2O
18.02 g H2O
1.361 g MgCl2 x 1 mol MgCl2 = .01429 mol MgCl2
95.21 g MgCl2
.09983 mol H2O = 7
0.01429
n = 7
Click Here For Extra Help.
Extra help with hydrates can be found here.
Have water molecules as part of their chemical formula
Contribute to the crystalline structure of the compound
HYDRATED vs ANHYDRIDES
Hydrated end up being ANHYDRIDES, which are chemicals that used to be hydrated.
Ordered (how they're arranged in space)=crystalline
What is the value of "n" for the hydrate of Magnesium Chloride H2O?
Mass of crucible = 22.130 g
Mass of crucible + hydrate = 25.290 g
Mass of crucible + contents after heating = 23.491 g
25.290 g (Cruc + Hyd) - 22.130 g (Cruc) = 3.160 (Hydrate)
23.491 g (after heating) - 22.130 g (crucible) = 1.361 g (Anhydrous)
25.290 g (hydrate) - 1.361 (anhydrous)
= 1.799 g H2O driven off
1.799 g H2O x 1 mol H2O = .09983 mol H2O
18.02 g H2O
1.361 g MgCl2 x 1 mol MgCl2 = .01429 mol MgCl2
95.21 g MgCl2
.09983 mol H2O = 7
0.01429
n = 7
Click Here For Extra Help.
Extra help with hydrates can be found here.
Thursday, November 5, 2015
Molar Mass
MOLAR MASS is the mass in grams of 1 mole of a substance
- unit= g/mol
- Gram Molecular Mass
- Covalent Compounds (no metals)
- Gram Formula Mass
- Ionic Compounds
JUMPING RIGHT IN...
- C3 H8
H 8 (# in compound) x 1.01 (Periodic Table) = 8.08
36.03 + 8.08 = 44.11 g/mol
DIATOMIC ATOMS
H2, O2, F2, Br2, I2, N2, Cl2
they only double their mass when ALONE
EX: O2 --> (2)(16.00)= 32.00 g/mol
For more Diatomic Atom help, Click Here.
Watch the video below, or check out this website for more help with molar mass calculations.
Watch the video below, or check out this website for more help with molar mass calculations.
(n.d.). Retrieved November 6, 2015, from https://d2gne97vdumgn3.cloudfront.net/api/file/T3WWBj9jQb67Siqvkqi5
(n.d.). Retrieved November 6, 2015, from http://study.com/academy/lesson/diatomic-molecule-definition-example.html
How to Calculate Molar Mass. (n.d.). Retrieved November 6, 2015, from https://www.youtube.com/watch?v=F9NkYSKJifs
(n.d.). Retrieved November 6, 2015, from http://study.com/academy/lesson/diatomic-molecule-definition-example.html
How to Calculate Molar Mass. (n.d.). Retrieved November 6, 2015, from https://www.youtube.com/watch?v=F9NkYSKJifs
Molar Mass of Compounds - Boundless Open Textbook. (n.d.). Retrieved November 6, 2015, from https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/mass-relationships-and-chemical-equations-3/molar-mass-41/molar-mass-of-compounds-223-7524/
Wednesday, November 4, 2015
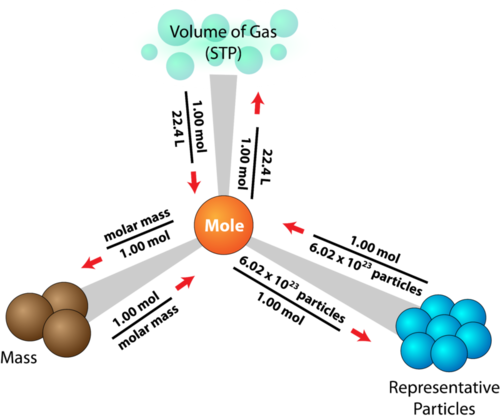
The Mole
THE MOLE and no, not the animal this time. The mole that we are going to talk about is a quantity of measurement, the 6.02 x 10^23
SOLVING MOLE PROBLEMS...
FOR EXAMPLE, How many moles of Mg is 3.01 x 10^22 atoms of Mg?
WELL,
3.01 x 10^22atoms Mg x 1 mol Mg = 5.00 x 10^-2 mol Mg
6.02 x 10^23atoms Mg
The Mole Highway Roadmap. (n.d.). Retrieved November 5, 2015.
(n.d.). Retrieved November 5, 2015, from https://dr282zn36sxxg.cloudfront.net/datastreams/f-d:869497f7016d9cb18cc1266f9c9d6edfc6149b50b3262dd16764aac7+IMAGE_THUMB_POSTCARD+IMA
SOLVING MOLE PROBLEMS...
- Decide what the question is asking
- Write down all the given information
- Label all quantities
- Solve the Equation
WELL,
3.01 x 10^22
6.02 x 10^23
The Mole Highway Roadmap. (n.d.). Retrieved November 5, 2015.
(n.d.). Retrieved November 5, 2015, from https://dr282zn36sxxg.cloudfront.net/datastreams/f-d:869497f7016d9cb18cc1266f9c9d6edfc6149b50b3262dd16764aac7+IMAGE_THUMB_POSTCARD+IMA
Subscribe to:
Comments (Atom)