The Mole
THE MOLE and no, not the animal this time. The mole that we are going to talk about is a quantity of measurement, the 6.02 x 10^23
SOLVING MOLE PROBLEMS...
- Decide what the question is asking
- Write down all the given information
- Label all quantities
- Solve the Equation
Let's look at the MOLE ROAD MAP to help us solve problems.
FOR EXAMPLE, How many moles of Mg is 3.01 x 10^22 atoms of Mg?
WELL,
3.01 x 10^22 atoms Mg x 1 mol Mg = 5.00 x 10^-2 mol Mg
6.02 x 10^23 atoms Mg
The Mole Highway Roadmap. (n.d.). Retrieved November 5, 2015.
(n.d.). Retrieved November 5, 2015, from https://dr282zn36sxxg.cloudfront.net/datastreams/f-d:869497f7016d9cb18cc1266f9c9d6edfc6149b50b3262dd16764aac7+IMAGE_THUMB_POSTCARD+IMA

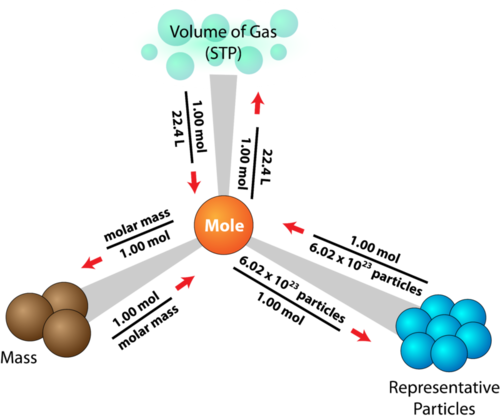
I really like the diagram you put up for the "mole triangle." The way it is presented here does not make it look that bad as it seemed in concept on the pretest.
ReplyDelete