I don't think that I did so well on the Gas Laws quiz. I know that for the test, I'll need to study the different laws closer, and notice the differences between them. I missed a day, and totally had no clue what STP was, which seems pretty important now.
STP
Tuesday, May 10, 2016
Air Bag Lab
We had to construct an air bag... one that would save our passenger's life but also not hurt the passenger from being too full of air.
Here's what we decided to do...
http://chemistry.about.com/od/chemicalreactions/f/What-Is-The-Equation-For-The-Reaction-Between-Baking-Soda-And-Vinegar.htm
Here's what we decided to do...
- Find maximum volume of the bag
- Fill bag with water
- pour H2O into a graduated cylinder--- Volume H2O=Volume CO2
- Calculate to determine amount of NaHCO3 needed
- Volume from step 1, convert to L
- Looking for NaHCO3... n=PV/RT
- solve for moles of CO2
- use stoich to find grams of NaHCO3
- Baking soda=vinegar 1:1 molar ratio
- Use D of vinegar to find volume of vinegar
- x.20 because vinegar is only 5% acetic acid
- Multiply measurements by .65 to not completely fill up the bag (or explode it)
http://chemistry.about.com/od/chemicalreactions/f/What-Is-The-Equation-For-The-Reaction-Between-Baking-Soda-And-Vinegar.htm
PVNRT
PV=nRT
R=universal gas constant .0821 L atm/ mol K
EX. Calculate the pressure in atm exerted by 1.82 mol of Sulfur Hexaflourine gas in a steel vessel with a volume of 5.43 L at 69.5 degrees C (+273.15)
PV=nRT
P= nRT/V
(1.82 x .0821 x 342.65)/ 5.43
=9.43 atm
http://www.phscale.net/idealgaslaw.htm
http://www.westfield.ma.edu/cmasi/gen_chem1/Gases/ideal%20gas%20law/pvnrt.htm
R=universal gas constant .0821 L atm/ mol K
PV=nRT
P= nRT/V
(1.82 x .0821 x 342.65)/ 5.43
=9.43 atm
http://www.phscale.net/idealgaslaw.htm
http://www.westfield.ma.edu/cmasi/gen_chem1/Gases/ideal%20gas%20law/pvnrt.htm
Measuring Pressure
Common units of pressure:
688mmHg x (1atm/760mmHg) = .905 atm
http://www.sengpielaudio.com/ConvPress.htm
- Pascal Pa
- Avg Air pressure at sea level 101,325
- Kilopascal kPa
- Avg Air pressure at sea level 101.325
- Atmosphere atm
- 1 exactly
- Millimeters of Mercury mmHg
- 760 exactly
- Inches of Mercury inHg
- 29.92
- Torr torr
- 760 exactly
- Pounds per square inch psi
- 14.7
688mmHg x (1atm/760mmHg) = .905 atm
http://www.sengpielaudio.com/ConvPress.htm
Boyle's Law
TELLS THE RELATIONSHIP BETWEEN PRESSURE AND VOLUME IN AN INVERSE RELATIONSHIP
P1V1=P2V2
Ideal gas at low pressure holds strictly to this relationship
http://www.wisegeek.org/what-is-boyles-law.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/gases-5/gas-laws-51/boyle-s-law-volume-and-pressure-254-8360/
P1V1=P2V2
Ideal gas at low pressure holds strictly to this relationship
http://www.wisegeek.org/what-is-boyles-law.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/gases-5/gas-laws-51/boyle-s-law-volume-and-pressure-254-8360/
Calculating Heat...
Q=MCΔT
Q= Heat in Joules
M= Mass in grams
C= Specific Heat
ΔT= Change in Temperature
EXAMPLE:
Calculate the amount of energy in joules required to heat 454 grams of water from 5.4 degrees C to 98.6 degrees C. Calculate the amount of energy in calories, too.
Q=?
M=454 g
Tinitial=5.4 degrees C
Tfinal=98.6 degrees C
c= 4.184 J/gdegC
Q=454x4.184x(98.6-5.4)
Q=1.77x10^5 Joules or 177 KJ
https://www.youtube.com/watch?v=4RkDJDDnIss
http://www.wikihow.com/Calculate-Specific-Heat
http://chemistry.about.com/od/workedchemistryproblems/a/Specific-Heat-Example-Problem.htm
Q= Heat in Joules
M= Mass in grams
C= Specific Heat
ΔT= Change in Temperature
EXAMPLE:
Calculate the amount of energy in joules required to heat 454 grams of water from 5.4 degrees C to 98.6 degrees C. Calculate the amount of energy in calories, too.
Q=?
M=454 g
Tinitial=5.4 degrees C
Tfinal=98.6 degrees C
c= 4.184 J/gdegC
Q=454x4.184x(98.6-5.4)
Q=1.77x10^5 Joules or 177 KJ
https://www.youtube.com/watch?v=4RkDJDDnIss
http://www.wikihow.com/Calculate-Specific-Heat
http://chemistry.about.com/od/workedchemistryproblems/a/Specific-Heat-Example-Problem.htm
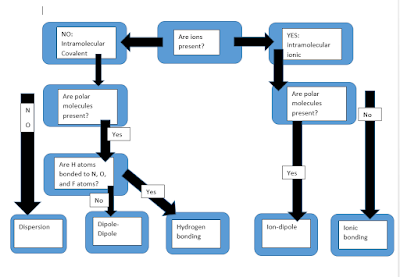
Intra/Inter Molecular Forces
I made this diagram to help with intramolecular and intermolecular forces... hope it's helpful...
http://chemed.chem.purdue.edu/genchem/topicreview/bp/intermol/intermol.html
Biodiesel Research...
Compilation of Biodiesel Research...
http://www.biodiesel.com/biodiesel/what-is-biodiesel/
http://www.fueleconomy.gov/feg/biodiesel.shtml
Click here for my favorite study music
- renewable
- clean-energy
- Blends of B20 can be used in a diesel engine without need for modifications
- IS registered with the Environmental Protection Agency in the United States
- Can be blended with petroleum or used purely
- Statistically safer than petroleum diesel
- made with natural vegetable oils and fats
http://www.biodiesel.com/biodiesel/what-is-biodiesel/
http://www.fueleconomy.gov/feg/biodiesel.shtml
Click here for my favorite study music
Monday, May 9, 2016
Making Biodiesel
WE MADE OUR OWN BIODIESEL
In class, we used Chickfila frier oil to create renewable biodiesel!
How to make biodiesel with used cooking oil
Everyone should make their own biodiesel.
http://biodiesel.org/
In class, we used Chickfila frier oil to create renewable biodiesel!
How to make biodiesel with used cooking oil
Everyone should make their own biodiesel.
http://biodiesel.org/
BIODIESEL
Biodiesel Video!!
Biodiesel is a cleaner fuel, and better for the environment. Our class made biodiesel videos to help spread the word about the renewable resource. Check out our videos!
OUR biodiesel video
another informative video...
This one just made us laugh :)
Biodiesel is a cleaner fuel, and better for the environment. Our class made biodiesel videos to help spread the word about the renewable resource. Check out our videos!
OUR biodiesel video
another informative video...
This one just made us laugh :)
Covalent Bonds
Result of sharing of electrons by two nonmetal atoms
BOTH atoms fill their octet (H exception)
Octet is satisfied when the two atoms are combined
http://quatr.us/chemistry/atoms/covalent.htm
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds
Covalent Bonding Video
BOTH atoms fill their octet (H exception)
Octet is satisfied when the two atoms are combined
http://quatr.us/chemistry/atoms/covalent.htm
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds
Covalent Bonding Video
Bonding
Electron Dot Formulas
-includes bonded and unbonded electron pairs
1) Calculate the total number of valence electrons by adding all of the valence electrons for each atom in the molecule
2) Divide the total valence electrons by 2 to find the number of electron pairs in the molecule
3) Surround the central atom with 4 electron pairs
Use the remaining electron pairs to complete the octet around the other atoms. H is the only exception, which only needs 2 electrons
4) Electron pairs that are shared by atoms are called bonding electrons
Others used to complete octets are lone pairs
5) If there are not enough electron pairs to provide each atom with an octet, move a nonbonding electron pair between two atoms that already share an electron pair
-includes bonded and unbonded electron pairs
1) Calculate the total number of valence electrons by adding all of the valence electrons for each atom in the molecule
2) Divide the total valence electrons by 2 to find the number of electron pairs in the molecule
3) Surround the central atom with 4 electron pairs
Use the remaining electron pairs to complete the octet around the other atoms. H is the only exception, which only needs 2 electrons
4) Electron pairs that are shared by atoms are called bonding electrons
Others used to complete octets are lone pairs
5) If there are not enough electron pairs to provide each atom with an octet, move a nonbonding electron pair between two atoms that already share an electron pair
Wednesday, March 9, 2016
Chemical Bonding
Chemical Bonding
Lewis Dot Structure/Electron placement
1. Use the valence electrons
2. Place the valence electrons around the symbol
EX: Nitrogen is 1s2 2s2 2p3, meaning that it has 5 valence electrons (Highest energy level=2, 2s2 & 2p3, 2+3 = 5)
This means that 5 electrons will need to be accounted for.
Covalent Bonds, a bond between two nonmetal atoms.
Bond length is the distance between two nuclei. Measurement between the radius of the bonding atoms is less than the sum of the radii added together.
More Complex Lewis Dot Problems...
Sulfur trioxide
SO3
Sulfur has 6 valence electrons
Oxygen has 6 valence electrons, but there are 3 oxygen, so 3x6=18
Sulfur valence + Oxygen valence = 24 e-
So we begin our structure...
O
l
O - S - O
And notice that there are three bonds, making six electrons accounted for. (2 per bond)
So that means out of our 24 e- we need to account for, 6 are taken care of just by bonds. However, there are still 18 e- that are unaccounted for. So we continue, adding electrons to the Oxygen...
. .
: O :
. . l . .
:O - S - O:
. . . .
HEY! It looks done. But it's not. Sure all 24 electrons are accounted for, but S only has 6 electrons, not 8. This means we can double bond an oxygen, and recognizing that there will be resonance, it will look like, and variations of this
. .
: O :
. . l . .
:O - S =O
. . . .
If you need a better tutorial, or a little more explanation, click here.
Lewis Dot Structure/Electron placement
1. Use the valence electrons
2. Place the valence electrons around the symbol
EX: Nitrogen is 1s2 2s2 2p3, meaning that it has 5 valence electrons (Highest energy level=2, 2s2 & 2p3, 2+3 = 5)
This means that 5 electrons will need to be accounted for.
Bond length is the distance between two nuclei. Measurement between the radius of the bonding atoms is less than the sum of the radii added together.
More Complex Lewis Dot Problems...
Sulfur trioxide
SO3
Sulfur has 6 valence electrons
Oxygen has 6 valence electrons, but there are 3 oxygen, so 3x6=18
Sulfur valence + Oxygen valence = 24 e-
So we begin our structure...
O
l
O - S - O
And notice that there are three bonds, making six electrons accounted for. (2 per bond)
So that means out of our 24 e- we need to account for, 6 are taken care of just by bonds. However, there are still 18 e- that are unaccounted for. So we continue, adding electrons to the Oxygen...
. .
: O :
. . l . .
:O - S - O:
. . . .
HEY! It looks done. But it's not. Sure all 24 electrons are accounted for, but S only has 6 electrons, not 8. This means we can double bond an oxygen, and recognizing that there will be resonance, it will look like, and variations of this
. .
: O :
. . l . .
:O - S =O
. . . .
If you need a better tutorial, or a little more explanation, click here.
Periodic Trends on the Exam
Periodic trends were on the exam, and when I saw the first question about them I freaked out. How was I supposed to know the answer to that? There's so many trends. But, I flipped over my handy dandy periodic table and wrote out the chart that I made for trends. THATS how I'm supposed to answer those questions. It took a lot of flipping back and forth of my periodic table to my chart, but I think ultimately that is what helped me the most on the exam. Point 1 for memorization.
Electron Configuration
Examples of electron configuration...
Li
1s2 2s1
O
1s2 2s2 2p4
Ar
1s2 2s2 2p6 3s2 3p6
OR Argon (looks like) [Ne] 3s2 3p6
http://www.chemistrytutorials.org/content/atomic-structure/electron-configuration
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations
Li
1s2 2s1
O
1s2 2s2 2p4
Ar
1s2 2s2 2p6 3s2 3p6
OR Argon (looks like) [Ne] 3s2 3p6
http://www.chemistrytutorials.org/content/atomic-structure/electron-configuration
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations
Electrons
2 electrons with the same spin will never occupy the same orbital...
N=1 1 Sublevel 1s Max. 2e-
N=2 2 sublevels 2s 2p max. 8e-
N=3 3 sublevels 3s 3p 3d max. 18 e-
N=4 4 sublevels 4s 4p 4d 4f max 32e-
AUFBAU- electrons enter orbitals of LOWEST energy FIRST
PAULI EXCLUSION- an orbital can only contain TWO electrons with opposite spin
HUND'S- within a sublevel electrons enter singly before pairing up
http://education.jlab.org/qa/atomicstructure_06.html
N=1 1 Sublevel 1s Max. 2e-
N=2 2 sublevels 2s 2p max. 8e-
N=3 3 sublevels 3s 3p 3d max. 18 e-
N=4 4 sublevels 4s 4p 4d 4f max 32e-
AUFBAU- electrons enter orbitals of LOWEST energy FIRST
PAULI EXCLUSION- an orbital can only contain TWO electrons with opposite spin
HUND'S- within a sublevel electrons enter singly before pairing up
http://education.jlab.org/qa/atomicstructure_06.html
4 Levels of Organization
1) Principal Energy Level---School Building
How far away from the nucleus an electron can be found (n)
2)Sublevel---Floor Number
(s, p, d, f)
1st principal energy level has one Sublevel, 1s
The 2nd principal energy level has 2 sublevels, 2s and 2p
The 3rd energy level has 3 sublevels, 3s, 3p, 3d
4th and all subsequent principal levels n=4s, 4p, 4d, 4f
http://w
ww.edu.pe.ca/kish/grassroots/chem/electron.htm
How far away from the nucleus an electron can be found (n)
2)Sublevel---Floor Number
(s, p, d, f)
1st principal energy level has one Sublevel, 1s
The 2nd principal energy level has 2 sublevels, 2s and 2p
The 3rd energy level has 3 sublevels, 3s, 3p, 3d
4th and all subsequent principal levels n=4s, 4p, 4d, 4f
http://w
ww.edu.pe.ca/kish/grassroots/chem/electron.htm
Trends in electronegativity
Electronegativity of an element is defined to be the tendency of an atom to draw electrons toward itself when chemically combined with another element. There are no units for this electronegativity, but it is just to be used as a comparative tool. Larger electronegativities pull electrons to themselves when bonded with other elements. Noble gases don't count when looking at electronegativity. Otherwise, electronegativity increases ⬆️➡️ The periodic table. http://www.chemguide.co.uk/atoms/bonding/electroneg.html
Periodic Trends
Again, the best way that I learn is through visuals.
Electronegativity increases ⬆️➡️ On the periodic table
Atomic Radius increases ⬇️⬅️ On the periodic table
Nonmetallic increases ↗️
Metallic increases ↙️
Electron affinity increases ⬆️➡️
Ionization energy increases ⬆️➡️ On the periodic table
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
Electronegativity increases ⬆️➡️ On the periodic table
Atomic Radius increases ⬇️⬅️ On the periodic table
Nonmetallic increases ↗️
Metallic increases ↙️
Electron affinity increases ⬆️➡️
Ionization energy increases ⬆️➡️ On the periodic table
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
What is ICE Box?
I missed the lecture on "Ice Box", but this is what I've learned so far. ICE stands for Initial Change Equilibrium. It's also easier for me to learn through visuals and example, so I mostly just have links of pictures to share. http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Equilibria/Le_Chatelier's_Principle/Ice_Tables
https://m.youtube.com/watch?v=tT-2xk9ZG_A
https://m.youtube.com/watch?v=tT-2xk9ZG_A
Equilibrium Help
Just a few extra help websites for equilibrium equations
http://scienceaid.co.uk/chemistry/physical/eqconstants.html
http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Equilibria/Chemical_Equilibria/The_Equilibrium_Constant/Balanced_Equations_And_Equilibrium_Constants_2
http://scienceaid.co.uk/chemistry/physical/eqconstants.html
http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Equilibria/Chemical_Equilibria/The_Equilibrium_Constant/Balanced_Equations_And_Equilibrium_Constants_2
ACID BASE
For acid base reactions, it's important to know how to determine pH and pOH of solutions. Basically, you're just finding the H+ or OH- concentration of the solution.
EX:
The pH of a 12.5 M solution of HCl
pH= -log [H+]
-log [12.5 M]
pH= -1.097
Thursday, February 18, 2016
Thursday, January 28, 2016
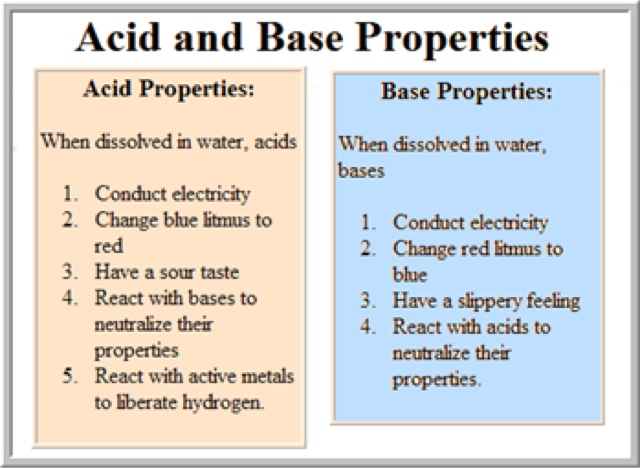
Acid and Bases
http://www.chem4kids.com/files/react_acidbase.html
http://www.chemtutor.com/acid.htm
BASES: taste bitter and feel slippery
ARRHENIUS ACIDS AND BASES
-Arrhenius acids are those species that produce hydrogen ions in solution (H+)
-Arrhenius bases are those species that produce hydroxide ions in solution (OH-)
HCl-->H+ + Cl- (strong acid)
NaOH-->Na+ + OH- (strong base)
WATER CAN BE AN ACID OR A BASE (amphoteric)
BRØNSTED-LOWREY ACIDS AND BASES
Brønstead-Lowery acids donate a proton (H+)
Brønstead-Lowery bases accept a proton (H+)
HCl + H2O --> H3O+ + Cl-
Acid-Conjugate Base Pair [HCl and Cl-]
Base-Conjugate Acid Pair [H2O and H3O]
EX: HClO4 and ClO4- because H+ + ClO4-
Tuesday, January 26, 2016
Juice Lab
Testing for Vitamin C Content In Beverages
TITRATION
For the lab, we placed 20 drops of juice (V8 Golden Goddess, Pear Nectar, Apple, Unsweet White Grapefruit, and a Standard Vitamin C Solution) into a test tube, along with 3 drops of starch. We added I2 drop by drop until the color of the solution changed to blue. We then recorded the number of drops it took to turn the solution blue.
Thursday, January 21, 2016
Learning to Dilute
Learning to Dilute
To simplify dilutions,
To simplify dilutions,
- Determine what you do and do not know
- Plug your values into the formula (M1V1=M2V2)
- Account for any differences in units
- Use simple algebra to solve the problem
- Round, keeping significant digits in mind.
I know it seems self explanatory, but it helps me when everything is listed out. Step-by-step is easiest!!
I was also taught the triangle method when working with problems.
In this method, if you were trying to solve for Liters, you would cover Liters on the triangle and are left with Moles of solute divided by molarity. In the same fashion, MxL=Mol and Mol/L=M
For more help Click: Dilutions and Triangle Method
Wednesday, January 13, 2016
WET LAB-Murder Lab
In our lab today, we discovered that the murder weapon was Silver Nitrate.
We reacted the AgNO3 with Na2CO3, forming a solid, and then we filtered out the liquid from the solid using a flask and filter paper. We have to let the product dry for a day or two, but check out our pictures!!
To do your own version of the lab, http://www.nclark.net/Honors_Molarity_Lab.doc
Or for molarity help,
Monday, January 11, 2016
Pre-Lab: Murder Mystery
Someone murdered Miss Scarlet! Our job is to determine the unknown poison, and the molarity of said poison, in order to fully determine which house guest killed Scarlet. Here's what we have so far:
Friday, January 8, 2016
Dilutions Lab
Dilutions Lab
Dilutions do not involve a chemical reaction.
M1V1=M2V2
While M is molarity and V is volume, M1 and V1 come from your stock solution, while M2 and V2 come from what you're making. However, the secondary volume=total solution, meaning aliquot + water. Aliquot=small sample of a solution
Seriel Dilutions=subsequent dilutions from stock solution
Subscribe to:
Comments (Atom)

.jpg)