Tuesday, December 15, 2015
Test Today
Good luck to everyone on the Stoichiometry test today. This unit is slightly easier than the last few, so don't stress too much, but know your stuff. The following link helped me study!
Monday, December 14, 2015
Stoichiometry Lab Overview
Beautiful Lab!
Click here for a demonstration: https://m.youtube.com/watch?v=f2X_8ViaDo8
After completing the lab, it is clear that we used Fe (III) rather than Fe (II). We made 1.8849 grams of Cu with a 85.17% yield.
Extra help:
Friday, December 11, 2015
Limiting Reagents
1. Balance the chemical equation for the chemical reaction
2. Convert the given information into moles.
3. Use stoichiometry for each individual reactant to find the mass of product produced
4. The reacant that produces a larger amount of product is the limiting reagent.
5. The reactant that produces a larger amount of product is the excess reagent
6. To find the amount of remaining excess reactant, subtract the mass of excess reagent consumed from the total mass of excess reagent given.
Extra problems:
LAB DAY 2!!
Lab day 2!!
At the end of the lab, our Fe was 6.4298 grams. So, 1.2968 grams of Fe reacted in the lab.
Fe(II) 1.2968gFe * 1molFe/55.85gFe * 1mol Cu/1molFe * 63.55gCu/1molCu =1.476gCu
Fe(III) 1.2968gFe* 1molFe/55.85gFe * 3molCu/2molFe * 63.55gCu/1molCu = 2.213gCu
Thursday, December 3, 2015
Cramming?
I know there's a lot of people cramming for the test. If you need any help with oxidation rules, I found this to be really helpful when I was studying!!
Wednesday, December 2, 2015
Reaction Overview
Studying for the test tomorrow, I thought it would be helpful to share a summary of reactions.
In a double replacement
It forms a solid
2 ionic compounds that are both aq
AB+CD->AD+BC
In Acid/Base
Form water
H+ +OH- as reactants
Bc+BOH->Bx+H2O
In Redox
Transfer of electrons
Single replacement
A+BC->AC+B
Combustion
Compound + O2 ->
Synthesis
A+B->C
Decomposition
C->A+B
Extra help:
https://m.youtube.com/watch?v=lQ6FBA1HM3s
REDOX Reactions Lab
Yesterday we conducted a lab with reactions using metals and solutions.
Included are pictures from the lab, showing the interesting reactions of metals when reacted with solutions. Many trials did not react, but those that did produced things such as bubbles and smoke.
Friday, November 27, 2015
Acid Base Reactions
Acid Base Reactions
In an Acid Base Reaction, the driving force is the production of water. These reactions will produce a salt and water as products. Salt= cation of base and anion of an acid
A solution of aq nitric acid and aq potassium hydroxide are mixed in a titration
Molecular: HNO3 (aq) + KOH (aq) --> KNO3 + H2O (l)
Net: H+ (aq) + OH- (aq) --> H2O (l)
Click here for extra help!
In an Acid Base Reaction, the driving force is the production of water. These reactions will produce a salt and water as products. Salt= cation of base and anion of an acid
A solution of aq nitric acid and aq potassium hydroxide are mixed in a titration
Molecular: HNO3 (aq) + KOH (aq) --> KNO3 + H2O (l)
Net: H+ (aq) + OH- (aq) --> H2O (l)
Click here for extra help!
Monday, November 23, 2015
Chemical Reactions
Chemical Reactions
Sensory Clues a change is taking place
Sensory Clues a change is taking place
- color has changed
- a solid forms
- bubbles form (in a liquid)
- Heat and/or a flame is produced, or heat is absorbed
Heat is Enthalpy = H
Delta H = Change in enthalpy
CHEMICAL REACTION LABS!
These are the pictures of products from our chemical reactions lab on Friday.
Friday, November 13, 2015
Empirical Formulas...
Empirical Formulas
What is % Composition of C10H14O?
Calculate the mass of H in 25.4 g of C10H14O.
C= 10 x 12.01 = 120.1
H= 14 x 1.01 = 14.14
O= 1 x 16 = 16.00
120.1 + 14.14 + 16.00 = 150.24 g/mol
%C= (120.1/150.24)(100)= 79.94% C
%H= (14.14/150.24)(100)= 9.412% H
%O= (16.00/150.24)(100)= 10.65% O
(9.412%)(25.4 g)= 2.39 g H
MOLECULAR FORMULAS
-whole number multiple of empirical formula
What is % Composition of C10H14O?
Calculate the mass of H in 25.4 g of C10H14O.
C= 10 x 12.01 = 120.1
H= 14 x 1.01 = 14.14
O= 1 x 16 = 16.00
120.1 + 14.14 + 16.00 = 150.24 g/mol
%C= (120.1/150.24)(100)= 79.94% C
%H= (14.14/150.24)(100)= 9.412% H
%O= (16.00/150.24)(100)= 10.65% O
(9.412%)(25.4 g)= 2.39 g H
MOLECULAR FORMULAS
-whole number multiple of empirical formula
- ratio of masses
-Can be the same as the empirical formula
EX: P4O10
EMPIRICAL FORMULAS
-lowest whole number ratio of elements in a compound
-start with % composition and work to moles
Thursday, November 12, 2015
Formula of a Chloride Lab
Formula of a Chloride Lab
Procedure:
Procedure:
- Determine the mass of a clean, dry 100 mL beaker to the nearest 0.001 g and record this mass on the data table
- Have your instructor place a small amount of zinc into the beaker. Then determine the mass of the beaker plus zinc and record in the data table.
- Using a graduated cylinder to measure, add 10 mL 3M HCl. Using a hot plate, gently heat the mixture until all of the zinc has dissolved
- Using a Bunsen burner, heat the solution until all of the water and excess HCl have been boiled away. Stop the heating as the last bit of liquid disappears. Do not continue heating after it has boiled to dryness as the compound will begin to decompose.
- Cool for several minutes and then determine the mass of the beaker and contents.
 |
| Be careful when working with Chlorine gas... You might end up not feeling well and watching the lab from the hall. |
Friday, November 6, 2015
Hydrated Compounds
Hydrated Compounds...
Have water molecules as part of their chemical formula
Contribute to the crystalline structure of the compound
HYDRATED vs ANHYDRIDES
Hydrated end up being ANHYDRIDES, which are chemicals that used to be hydrated.
Ordered (how they're arranged in space)=crystalline
What is the value of "n" for the hydrate of Magnesium Chloride H2O?
Mass of crucible = 22.130 g
Mass of crucible + hydrate = 25.290 g
Mass of crucible + contents after heating = 23.491 g
25.290 g (Cruc + Hyd) - 22.130 g (Cruc) = 3.160 (Hydrate)
23.491 g (after heating) - 22.130 g (crucible) = 1.361 g (Anhydrous)
25.290 g (hydrate) - 1.361 (anhydrous)
= 1.799 g H2O driven off
1.799 g H2O x 1 mol H2O = .09983 mol H2O
18.02 g H2O
1.361 g MgCl2 x 1 mol MgCl2 = .01429 mol MgCl2
95.21 g MgCl2
.09983 mol H2O = 7
0.01429
n = 7
Click Here For Extra Help.
Extra help with hydrates can be found here.
Have water molecules as part of their chemical formula
Contribute to the crystalline structure of the compound
HYDRATED vs ANHYDRIDES
Hydrated end up being ANHYDRIDES, which are chemicals that used to be hydrated.
Ordered (how they're arranged in space)=crystalline
What is the value of "n" for the hydrate of Magnesium Chloride H2O?
Mass of crucible = 22.130 g
Mass of crucible + hydrate = 25.290 g
Mass of crucible + contents after heating = 23.491 g
25.290 g (Cruc + Hyd) - 22.130 g (Cruc) = 3.160 (Hydrate)
23.491 g (after heating) - 22.130 g (crucible) = 1.361 g (Anhydrous)
25.290 g (hydrate) - 1.361 (anhydrous)
= 1.799 g H2O driven off
1.799 g H2O x 1 mol H2O = .09983 mol H2O
18.02 g H2O
1.361 g MgCl2 x 1 mol MgCl2 = .01429 mol MgCl2
95.21 g MgCl2
.09983 mol H2O = 7
0.01429
n = 7
Click Here For Extra Help.
Extra help with hydrates can be found here.
Thursday, November 5, 2015
Molar Mass
MOLAR MASS is the mass in grams of 1 mole of a substance
- unit= g/mol
- Gram Molecular Mass
- Covalent Compounds (no metals)
- Gram Formula Mass
- Ionic Compounds
JUMPING RIGHT IN...
- C3 H8
H 8 (# in compound) x 1.01 (Periodic Table) = 8.08
36.03 + 8.08 = 44.11 g/mol
DIATOMIC ATOMS
H2, O2, F2, Br2, I2, N2, Cl2
they only double their mass when ALONE
EX: O2 --> (2)(16.00)= 32.00 g/mol
For more Diatomic Atom help, Click Here.
Watch the video below, or check out this website for more help with molar mass calculations.
Watch the video below, or check out this website for more help with molar mass calculations.
(n.d.). Retrieved November 6, 2015, from https://d2gne97vdumgn3.cloudfront.net/api/file/T3WWBj9jQb67Siqvkqi5
(n.d.). Retrieved November 6, 2015, from http://study.com/academy/lesson/diatomic-molecule-definition-example.html
How to Calculate Molar Mass. (n.d.). Retrieved November 6, 2015, from https://www.youtube.com/watch?v=F9NkYSKJifs
(n.d.). Retrieved November 6, 2015, from http://study.com/academy/lesson/diatomic-molecule-definition-example.html
How to Calculate Molar Mass. (n.d.). Retrieved November 6, 2015, from https://www.youtube.com/watch?v=F9NkYSKJifs
Molar Mass of Compounds - Boundless Open Textbook. (n.d.). Retrieved November 6, 2015, from https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/mass-relationships-and-chemical-equations-3/molar-mass-41/molar-mass-of-compounds-223-7524/
Wednesday, November 4, 2015
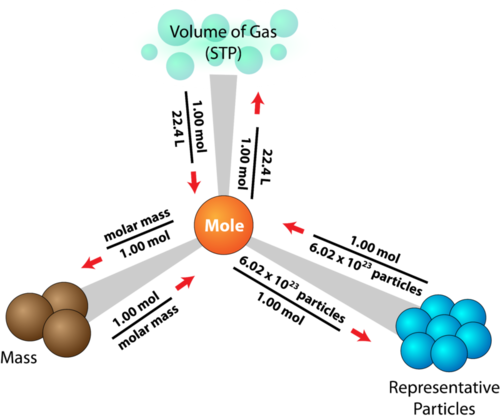
The Mole
THE MOLE and no, not the animal this time. The mole that we are going to talk about is a quantity of measurement, the 6.02 x 10^23
SOLVING MOLE PROBLEMS...
FOR EXAMPLE, How many moles of Mg is 3.01 x 10^22 atoms of Mg?
WELL,
3.01 x 10^22atoms Mg x 1 mol Mg = 5.00 x 10^-2 mol Mg
6.02 x 10^23atoms Mg
The Mole Highway Roadmap. (n.d.). Retrieved November 5, 2015.
(n.d.). Retrieved November 5, 2015, from https://dr282zn36sxxg.cloudfront.net/datastreams/f-d:869497f7016d9cb18cc1266f9c9d6edfc6149b50b3262dd16764aac7+IMAGE_THUMB_POSTCARD+IMA
SOLVING MOLE PROBLEMS...
- Decide what the question is asking
- Write down all the given information
- Label all quantities
- Solve the Equation
WELL,
3.01 x 10^22
6.02 x 10^23
The Mole Highway Roadmap. (n.d.). Retrieved November 5, 2015.
(n.d.). Retrieved November 5, 2015, from https://dr282zn36sxxg.cloudfront.net/datastreams/f-d:869497f7016d9cb18cc1266f9c9d6edfc6149b50b3262dd16764aac7+IMAGE_THUMB_POSTCARD+IMA
Thursday, October 29, 2015
Measurement Test
HONESTLY, I found the measurement test to be quite difficult. The test included dimensional analysis, a bit from Nomenclature, and a lot about significant digits. Remember that with significant digits, multiplication/division and addition/subtraction have different rules, and they can be difficult to follow when there is a large problem concerning the order of operations. Also, with dimensional analysis, be careful when you have a measured amount. I ran into trouble last night because I was rounding when I shouldn't have been on my Last Meal Conversion Project, and when you're using specific amounts, as in a recipe, you can't make things up. Make sure you're careful, and I'm praying for everyone else that says they also struggled with the exam today.
Wednesday, October 28, 2015
QUIZ
Our Measurement quiz went quite well, we are expecting a lot of As and Bs this time around. The significant figures questions were really tricky because you had to pay close attention to what was going on. There are different rules for multiplying/dividing and adding/subtracting, so when the order of operations was to be used, you really have to pay attention. Good luck to everyone expecting their grades soon!!
Dimensional Analysis
Dimensional Analysis
Summation of Yesterday's Notes
DIMENSIONAL ANALYSIS
-Convert 1 quantity to another
Infinite Sig Figs occur when
-you have a counted quantity
-you have an exact measurement
NEVER START WITH A CONVERSION FACTOR
UNITS HAVE TO CANCEL LEFT TO RIGHT
Ex:
8.00 m 100 cm 1 in
_______ x ________ x _______ = 315 inches
1 1 m 2.54 cm
Extra Notes:
Temperature: Average Kinetic Energy of the particles in a sample
K='C + 273.15
In Kelvin temperatures, there is no negative.
0 energy = 0 temperature
'F= 9/5 ('C) +32
'C= 5/9 ('F-32)
Thursday, October 22, 2015
Significant Figures
Significant Figures...
So, today in the wonderful world of pre-ap chemistry, we learned all about significant figures. It's actually not as hard as it looks... once you finally get it down. Allow me to explain.Rule #1) You can estimate 1 digit past the calibration on the instrument used.
Rule #2) understand that significant digits indicate accuracy, not quantity.
Rule #3) When rounding, round 4 and below down, and 5 or above up.
Rule #4) The last digit in a measurement is ALWAYS uncertain.
Learning Through Example...
500 mLHas 1 significant digit... the 5
560 mL
Has 2 significant digits, the 5 and the 6
555.5
Has 4 significant digits.
0.004004500
The two zeros following the decimal, 0.004004500 are not significant, and their only purpose is to hold the place for the larger number as a decimal.
The remaining numbers are significant, but we will focus on these for now. 0.004004500, the two 4's and the 5 are significant because they are actually digits.
The two zeros that are locked in between the numerals, 0.004004500, are significant because of their position in being locked in.
The two zeros trailing at the end of the number, 0.004004500 are significant because they show accuracy within the number.
Comment if you have questions!! It's a little confusing at first, but the more examples, the better!!
References:
(n.d.). Retrieved October 23, 2015, from https://jahschem.wikispaces.com/file/view/sig-figs-examples.png/445639706/sig-figs-examples.png
Thursday, October 8, 2015
My Stuffed Mole
My Stuffed Mole
For our Mole Day project, I created a stuffed mole. His name is Penelope, and he has a pink pom-pom for a nose. Unfortunately, his mermaid costume will not be complete until later in the month. Please enjoy this photo of my mole interrupting a study session in Chemistry class.
For our Mole Day project, I created a stuffed mole. His name is Penelope, and he has a pink pom-pom for a nose. Unfortunately, his mermaid costume will not be complete until later in the month. Please enjoy this photo of my mole interrupting a study session in Chemistry class.
Wednesday, October 7, 2015
Aspirin Lab Group 2
Group 2 Aspirin Lab
For the second group of the Aspirin Lab, we had one group that tested into the lab. Hopefully they pull through for the rest of us and make enough aspirin for the rest of our labs. When making aspirin, the mixture should be left in the boiling water for 15 minutes, and ICE water must be added to the reaction mixture after it cools for 3 minutes. This is because the warmer the water, the faster the reaction will occur. For a controlled reaction, adding ICE water will be fast and will also not explode all over and kill us all. Be Safe and wear goggles if you are participating in lab!
For the second group of the Aspirin Lab, we had one group that tested into the lab. Hopefully they pull through for the rest of us and make enough aspirin for the rest of our labs. When making aspirin, the mixture should be left in the boiling water for 15 minutes, and ICE water must be added to the reaction mixture after it cools for 3 minutes. This is because the warmer the water, the faster the reaction will occur. For a controlled reaction, adding ICE water will be fast and will also not explode all over and kill us all. Be Safe and wear goggles if you are participating in lab!
Tuesday, October 6, 2015
Beginning of Mole Day
The Beginning of My Mole Day Make-A-Mole
I'm really excited about the Mole Day mole project. October 23 is Mole Day, and we are making stuffed Moles to celebrate. My Mole is going to be a pun-based Little Mole-Maid. My stuffed mole is going to have a pink pom pom nose and pink felt feet. I purchased fabric that is similar to animal fur, so my mole is not necessarily going to be realistic, but maybe it will at least feel realistic. My mole is going to have a detachable wig and mermaid tail for its Halloween costume and Mole-Day theme. I can't wait to finish up this project! Happy almost Mole Day.
| European Mole, courtesy of: (n.d.). Retrieved October 6, 2015, from http://designoneinc.com/wp-content/uploads/2013/12/european_mole_2.jpg |
Monday, October 5, 2015
Aspirin Lab Group 1
Group 1 Aspirin Lab
Nobody in group 1 passed the pre-lab quiz. The first round of the aspirin lab was therefore not conducted. This is what happens when the class as a whole does not study and understand the lab. The second group will likely conduct the experiment on Wednesday, seeing as more than just I study. Wishing luck to everyone on their pre-lab quiz on Wednesday.
Nobody in group 1 passed the pre-lab quiz. The first round of the aspirin lab was therefore not conducted. This is what happens when the class as a whole does not study and understand the lab. The second group will likely conduct the experiment on Wednesday, seeing as more than just I study. Wishing luck to everyone on their pre-lab quiz on Wednesday.
Friday, October 2, 2015
Test
Test
The test was so much easier than the pre-test. I really think that I did good on it. Hopefully, I get a good grade, I studied super hard. The test included some things from Nomenclature. There was ionic naming along with average atomic mass and finding the missing element. Alpha, Gamma, and Beta decay were all included in the test. Praying for a good grade.
The test was so much easier than the pre-test. I really think that I did good on it. Hopefully, I get a good grade, I studied super hard. The test included some things from Nomenclature. There was ionic naming along with average atomic mass and finding the missing element. Alpha, Gamma, and Beta decay were all included in the test. Praying for a good grade.
Thursday, October 1, 2015
Decay Activity
Decay Activity
To demonstrate half-lives, we cut up a piece of paper into 567 tiny squares. we put the squares in a cup and shook it up. If the paper landed on the color side, we counted them as decayed particles. This went on for quite a while until we had barely any active particles left. It was a very helpful tool to learn half-lives.
To demonstrate half-lives, we cut up a piece of paper into 567 tiny squares. we put the squares in a cup and shook it up. If the paper landed on the color side, we counted them as decayed particles. This went on for quite a while until we had barely any active particles left. It was a very helpful tool to learn half-lives.
Tuesday, September 22, 2015
Beanium Lab
Beanium Lab
Today in class we did a "Beanium Lab" pretending that beans were a new element looking at the different isotopes. We uses white, black, red, and pinto beans for this "experiment". We found that in our sample of Beanium, we had 18 white isotopes, 12 black, 10 red, and 13 pinto, for a total of 53 isotopes. We discovered that the total mass of the white beanium was 5.45 grams, black was 2.36 grams, red was 3.43 grams, and pinto was 4.20 grams. The average mass of the isotope was .303 g white, 0.197 g black, 0.343 g red, and 0.323 g pinto. We also calculated the abundance of each isotope, with white being 33.96%, black being 22.64%, red being 18.87%, and pinto 24.53% abundance. Using the average mass of each isotope, the atomic mass formula, and the % abundance, to end this experiment, we calculated the atomic mass, which ended up being roughly 0.291 grams.
Reference:
(n.d.). Retrieved September 22, 2015, from http://www.pulsecanada.com/uploads/b6/1n/b61n7chYD_ubrx8ybPToGA/pinto.jpg
Today in class we did a "Beanium Lab" pretending that beans were a new element looking at the different isotopes. We uses white, black, red, and pinto beans for this "experiment". We found that in our sample of Beanium, we had 18 white isotopes, 12 black, 10 red, and 13 pinto, for a total of 53 isotopes. We discovered that the total mass of the white beanium was 5.45 grams, black was 2.36 grams, red was 3.43 grams, and pinto was 4.20 grams. The average mass of the isotope was .303 g white, 0.197 g black, 0.343 g red, and 0.323 g pinto. We also calculated the abundance of each isotope, with white being 33.96%, black being 22.64%, red being 18.87%, and pinto 24.53% abundance. Using the average mass of each isotope, the atomic mass formula, and the % abundance, to end this experiment, we calculated the atomic mass, which ended up being roughly 0.291 grams.
| "Beanium" |
Reference:
(n.d.). Retrieved September 22, 2015, from http://www.pulsecanada.com/uploads/b6/1n/b61n7chYD_ubrx8ybPToGA/pinto.jpg
Monday, September 21, 2015
Isotopes
Isotopes
Naming isotopes...
A
X
Z
A is the mass number, which is the sum of protons and neutrons
Z is the atomic number, or the number of protons
X is the element symbol
1. Determine the number of protons
2. Determine the number of electrons
ADJUST ELECTRON COUNTS FOR IONS
3. Determine the number of neutrons
For Example:
References:
(n.d.). Retrieved September 21, 2015, from http://www.expertsmind.com/CMSImages/606_Nuclear Notation 1.png
Thursday, September 17, 2015
Day 1 of Atomic Structure
Atomic Structure
Yesterday, we learned about Dalton's Atomic Theory, and that not all of his theory is still true today. We practiced calculating the percent composition, also known as the proportion of mass. Thomson used a cathode ray tube in order to show atoms of any element emit particles with a negative charge. We learned about Rutherford and his gold foil, he gave us the proton. The current Atomic Model is a "cloud model", meaning that there are no distinctive boundaries.
Yesterday, we learned about Dalton's Atomic Theory, and that not all of his theory is still true today. We practiced calculating the percent composition, also known as the proportion of mass. Thomson used a cathode ray tube in order to show atoms of any element emit particles with a negative charge. We learned about Rutherford and his gold foil, he gave us the proton. The current Atomic Model is a "cloud model", meaning that there are no distinctive boundaries.
Atomic Structure and Radioactivity Pretest...
We recently took the Atomic Structure and Radioactivity Unit Pretest. It was difficult because I do not yet understand any of the fusing, fission, bonding, etc rules. Although we touched on half-lives way back when in physical science, I think I did alright on some of those questions. I still need a refresher before a final grade. Can't wait to learn more about this unit, it will be a good one!
Monday, September 14, 2015
Final, Final Thoughts about Nomenclature...
Finally, the information sticking with me is all about ionic compounds. I learned how to name them, even with roman numerals. We learned that cations are metals, and anions are nonmetals.
Final Thoughts on Nomenclature...
In the Nomenclature unit, the best thing that I did was the Frontier Chemistry Project. I'm in the woods and prairies a lot (truthfully) for running and hiking. I think that if I ever encountered some type of plant, I may be able to use it to my benefit, in case of an emergency. I've been very confident in identifying plants when I see them, and they can now be of help. This project not only helped me understand HOW the plants could help, but also WHY they'll help chemically.
Thursday, August 20, 2015
Introduction Page
| http://apcg.space.noa.gr/upload/lib/photos/infrastructure/acl2.jpg |
| http://www.centraltexasequinevet.com |
| http://i.huffpost.com |
Subscribe to:
Comments (Atom)